PATHOPHYSIOLOGY OF CUTANEOUS INFLAMMATION
Experimental models and effects of psychological stress.
Pierre Saint-Mezard
This work as been done in the laboratory of Pr Jean-Francois Nicolas, INSERM U503, IFR 128, Lyon, FRANCE.
Inflammation is a complex mechanism of interactions betweens soluble factors and cells, which occurs in all tissues in response to trauma, infectious pathogens (virus, bacteria, parasites), toxics, or after ischemic or autoimmune disorders. In general, this tissular reaction induces the clearance of pathogens and wound healing of the damaged organ. However, if this inflammatory reaction is uncontrolled, either by excessive response against the tissular target, or because of an abnormal wound healing, damages persist and lead to different degenerative diseases such as rheumatoid arthritis, diabetes, psoriasis or contact dermatitis.
Cutaneous inflammatory diseases such as contact dermatitis, are good models for the assessment of mechanisms involved in the antigen specific inflammation. First, a mouse model of contact dermatis called Contact Hypersensitivity (CHS) has been developed. Second, skin is an organ easily accessible for the study of the numerous factors responsible for inflammation.
The onset and the control of the cutaneous inflammation involve directly cells and mediators from the skin immune systems but also from the central nervous systems, which can regulate the inflammatory response in a reflex way or under stress conditions.
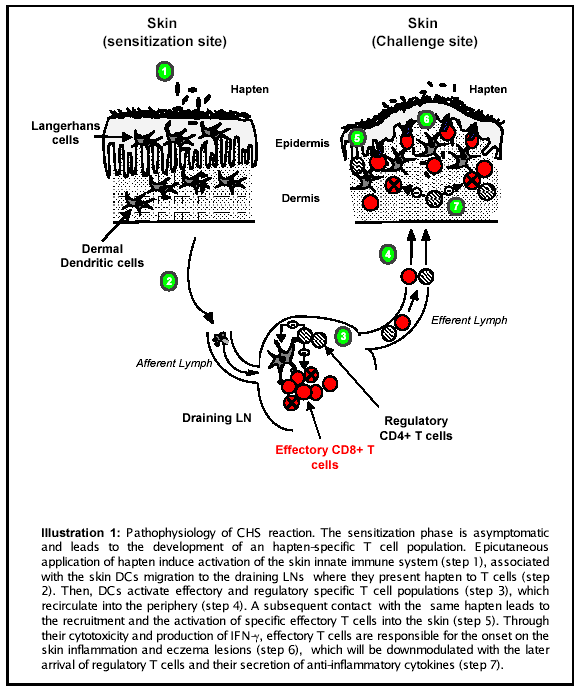
Even there are some histological differences regarding the species, two phases are classicaly described during the CHS reaction. The first enconter with the antigen, also called allergen, correspond to the sensitization phase. This stage is totally asymptomatic and leads to the generation of an allergen specific T cell population. During a further cutaneous exposure to the same allergen, specific T cells activation induces the elicitation phase, through the reruitment of inflammatory cells to the site of allergen location. This influx of macrophages and neutrophils is associated with a cutaneous inflammation which clinical signs can be visible during 24 to 96h. Illustration 1 summarized the pathophysiology of CHS as it is generally accepted (1). However, several effectory and regulatory mechanisms are still poorly understood in this inflammatory disease.

This PhD work had tried to better understand in a first part, mechanisms of effectory cells recruitment into the skin, and ways of control of their effectory functions. First, we showed that CHS can be induced in mouse after a single skin contact with haptens , explaining the « active sensitization » phenomenon described in human. This new model present a similar pathophysiology as the classical CHS reaction, but allow a different approach in the study of T cells recruitment into the skin (2). In an another study, we demonstrated that IL-18 in necessary for an efficient recruitment of specific T cells in the skin. Moreover, we point out the therapeutic potential of IL-18 binding protein (IL-18BP), a human protein able to neutralise the biological effect of IL-18 (3). Indeed, a serial subcutaneous injection of 250mg of IL-18BP during the elicitation phase is sufficient to down modulate significantly the CHS reaction (Illustration 2).
In a third work we focused our interest on the regulation of the CHS responses through the study of the CHS response in CD4 deficient mice. We described an MHC class II restricted T cell population endowed with strong peculiar regulatory properties. This result clarify the opposite results obtained from the CD4 and MHC class II deficient mice and confirm the effectory role of cytotoxic CD8 T cells (4).

In a second part, we developed an in vivo model where an acute psychological stress increase the CHS reaction. Mice were first submitted to a restraint stress during 2h30min and then sensitized with a suboptimal dose of haptens (0.2% DNFB, 0.5% FITC). Although no further stress was applied, the previously stressed mice developed 5 days later an enhanced inflammatory response compare to non-stressed control animals (Illustration 3A). This model allowed us to come up with a more detailed study of the modulation of the immune system function during activation of the CNS (5). We showed that the release of norepinephrine by symphatic fibers during acute stress, induced an increased migration of DC from the skin to draining LNs (Illustration 3B, 3C). This observation is expressed by an enhanced density of activated antigen-presenting-cells into the T cells area of the draining LN and then, by an enhanced availability of the Ag (signal 1) and costimulatory molecules (signal 2). This phenomenon is responsible for the increase of allergen specific cytotoxic T cell priming in vivo and reflect the adjuvant effect of stress. This findings provide new insights into the role of the CNS in the onset of inflammatory immune responses.

The study of the pathophysiology of the CHS reaction and the influence of psychological stress allowed us to study several aspects of the control and the onset on antigen specific inflammatory responses. Through different works, we provided new evidences on the complex mechanism of recruitment of effector T cells into the skin, clarified the paradoxical hapten-specific T cell responses into the CD4 deficient mice, and unveiled a part of the multiple functional interactions between the immune and central nervous systems. In conclusion, this work suggest new therapeutic ways through the use of IL-18BP as a treatment of Contact Dermatitis in human.
References:
1- Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, Niolas JF. Allergic contact dermatitis. Eur J Dermatol. 2004 14(5):284-95.
2- Saint-Mezard P, Krasteva M, Chavagnac C, Bosset S, Akiba H, Kehren J, Kanitakis J, Kaiserlian D, Nicolas JF, Berard F. Afferent and efferent phases of allergic contact dermatitis (ACD) can be induced after a single skin contact with haptens: evidence using a mousemodel of primary ACD. J Invest Dermatol. 2003 Apr;120(4):641-7.
3- Plitz T, Saint-Mezard P, Satho M, Herren S, Waltzinger C, de Carvalho Bittencourt M, Kosco-Vilbois MH, Chvatchko Y. IL-18 binding protein protects against contact hypersensitivity. J Immunol. 2003 Aug 1;171(3):1164-71.
4- Saint-Mezard P, Chavagnac C, Vocanson M, Rosieres A, Kehren J, Bosset S, Kaiserlian D, Nicolas JF, Berard F. CD4-deficient mice develop impaired class I-restricted but normal class II-restricted skin delayed type hypersensitivity reactions. J Invest Dermatol. 2005 124(3):562-9.
5- Saint-Mezard P, Chavagnac C, Bosset S, Ionescu M, Peyron E, Kaiserlian D, Nicolas JF, Berard F. Adjuvant effect of psychological stress on dendritic cell function in vivo. J Immunol. 2003 Oct 15;171(8):4073-4080.