Mitochondrial Uncoupling Protein UCP2:
intracellular signaling and metabolism in macrophages
Yalin EMRE
Thesis supervised by Anne-Marie CASSARD-DOULCIER at Université Descartes Paris 5 – Necker / CNRS UPR 9078 directed by Daniel RICQUIER.
Uncoupling protein UCP2 belongs to the family of mitochondrial carriers. Its biochemical function is still under debate. UCP2 expression is restricted to some tissues such as spleen, stomach or intestine. UCP2 is particularly present in macrophages where it regulates the production of reactive oxygen species (ROS).
Analysis of Ucp2-KO mice showed their better resistance to an infection by Toxoplasma gondii than wild-type animals thanks to highly active macrophages in terms of ROS production. In a murine model of human atherosclerosis, Ucp2-KO mice developed increased atherosclerotic lesions. Plaques in Ucp2-KO animals contained much more macrophages and nitric oxide (NO)-induced damage.
My thesis work was mainly focused on the mechanisms involved in the modulation of immune responses by UCP2.
1/ UCP2 controls MAPK signaling in macrophages
We first compared the activity of Ucp2-KO and Ucp2-WT macrophages. Ucp2-KO macrophages produced higher levels of pro-inflammatory cytokines and of NO in response to lipopolysaccharides (LPS) and had better transmigration ability compared to Ucp2-WT macrophages (ref 1).
ROS signaling is a critical event in macrophages activation. Since UCP2 regulates the production of ROS, we wondered whether UCP2 could be involved in signaling pathways activated in response to LPS.
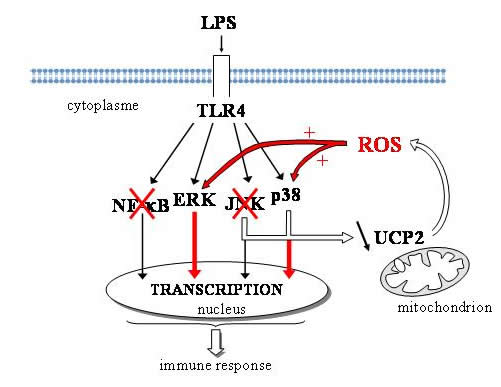
Analysis of MAPK and NF- k B signaling pathways led us to propose a model putting mitochondria in the heart of a signal amplification loop involving UCP2, ROS and MAPK (ref 1, figure 1). More precisely we identified UCP2 as a physiological break on LPS-induced ROS signaling. Upon stimulation, UCP2 is quickly downregulated through JNK and p38 pathways. The downregulation of UCP2 potentiates ERK and p38 activation via the increase of mitochondrial ROS production. As a consequence, signaling and activation of Ucp2-KO macrophages is accelerated, responsible of the increased activity of Ucp2-KO macrophages. |
 |
The relevance of these results was next investigated in vivo in the framework of infection and autoimmunity .
2/ Absence of UCP2 favors anti-listerial response
Mice infection with Listeria monocytogenes revealed a better resistance of Ucp2-KO mice (ref 2). Enhanced recruitment of macrophages and neutrophils was observed in the spleen of Ucp2-KO mice, accompanied by a higher ROS production. Moreover higher production of pro-inflammatory cytokines (IL-1 b , IL-6, IFN- g ) and of MCP-1 was detected in Ucp2-KO mice after 4 days of infection, preceded by a decrease of the anti-inflammatory cytokine IL-10 production. These data highlight the regulatory function of UCP2 on innate immunity.
3/ A bsence of UCP2 accelerates the development autoimmune diabetes
Infiltration of immune cells (macrophages and lymphocytes) into pancreatic islets of Langerhans and selective destruction of insulin-secreting b -cells are characteristics of type 1 diabetes.
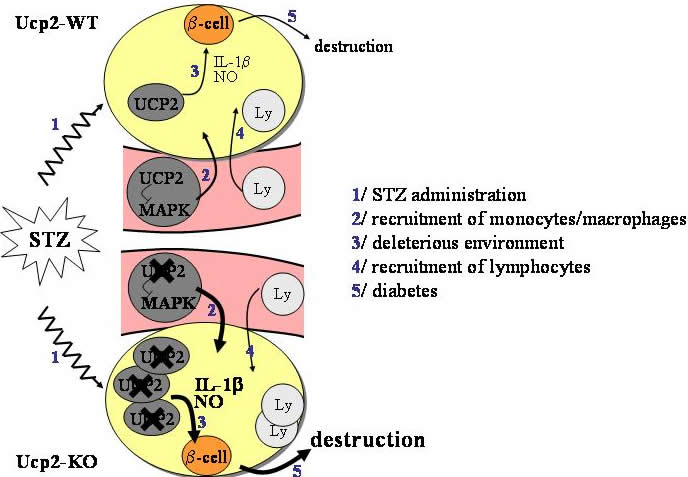
Using the multiple low-dose of streptozotocin (STZ) administration model, we discovered that the development of type 1 diabetes is strongly accelerated in Ucp2-KO mice compared to the WT with increased intra-islet lymphocytic infiltration (ref 3). In serum and pancreas, levels of IL-1 b were 3 to 5-fold increased in STZ-treated Ucp2-KO mice. Macrophages from STZ-treated Ucp2-KO mice had increased IL-1 b and nitric oxide (NO) production, compared with WT macrophages. In the same time, more macrophages were recruited into islets of STZ-treated Ucp2-KO mice. This was accompanied by increased NO/ROS-induced damage. Our results show that inflammation is stronger in Ucp2-KO mice. To be more precise, macrophage-mediated immunity is increased in Ucp2-KO mice , leading to the exacerbated disease in these mice (figure 2) . |
 |
Our work highlighted the mitochondrial protein UCP2 as a new player in macrophage signaling. UCP2 therefore constitutes an interesting target in the framework of inflammatory or autoimmune diseases, such as type 1 diabetes.
References :
(1) Emre Y, Hurtaud C, Nübel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM (2007) Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages.Biochem J 402:271-8.
(2) Rousset S, Emre Y, Join-Lambert O, Hurtaud C, Ricquier D, Cassard-Doulcier AM (2006) The uncoupling protein 2 modulates the cytokine balance in innate immunity.
Cytokine 35:135-42.
(3) Emre Y, Hurtaud C, Karaca M, Nubel T, Zavala F, Ricquier D (2007) Role of uncoupling protein UCP2 in cell-mediated immunity: how macrophage-mediated insulitis is accelerated in a model of autoimmune diabetes. Proc Natl Acad Sci USA 104:19085-90.