Characterization of C1q ligands implicated in apoptotic cell clearance
Helena Païdassi
This work has been done in the molecular enzymology laboratory of the Institute for Structural Biology (UMR 5075) in Grenoble, under supervision of Philippe Frachet
Apoptosis is a physiological form of programmed cell death. During apoptosis, dying cells acquire new cell surface determinants (also called « eat-me » signals). Specific recognition of these « eat-me » signals by professional phagocytes s is responsible for fast and efficient removal of apoptotic corpses, as well as for induction of appropriate immune response. In steady state, it prevents the development of inappropriate inflammatory reaction and maintains self tolerance (1).
Recent studies in the field have highlighted the implication of C1q in apoptotic cell removal and recognition. C1q is the recognition molecule of the complement classical pathway. In fact, C1q deficient patients develop lupus, an inflammatory autoimmune disease often associated with defects in apoptotic cell clearance.
C1q is a hexamer of heterotrimers which consists of two typical regions, a collagenous-like fragment (CLF) from which emerge six globular regions (GR). It has been indirectly demonstrated that GR are involved in the specific recognition of apoptotic cells. On the other hand, CLF are involved in C1q recognition at the surface of phagocytes (2). When I started my PhD, the phagocytic receptors for C1q had already been described, however, none of the ligands for C1q on the surface of apoptotic cells was known. Therefore, during my PhD Iset out to identify and characterize ligands exposed on dying cell surface during apoptosis, responsible for GR binding and thus for specific recognition of apoptotic cells by C1q.
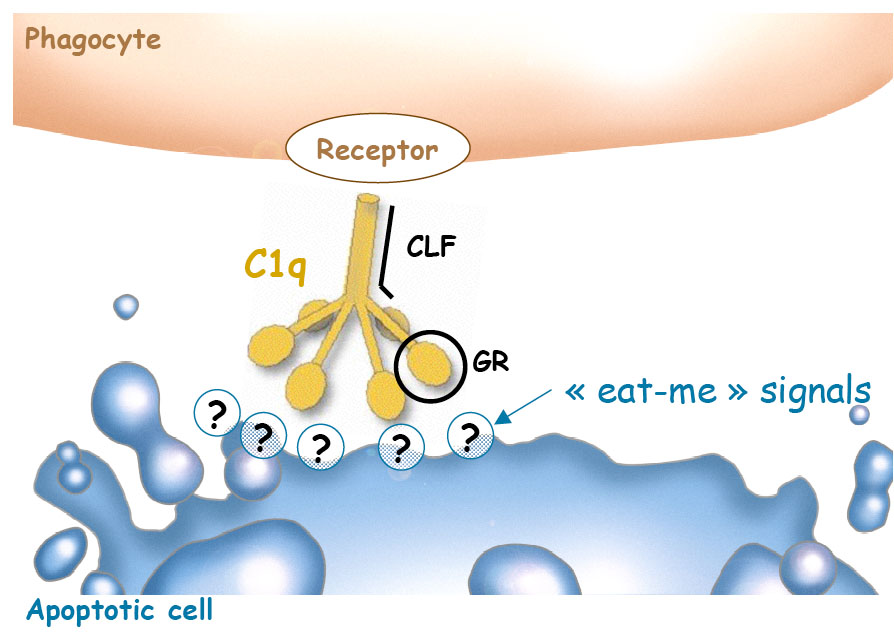
Role of C1q in apoptotic cell recognition
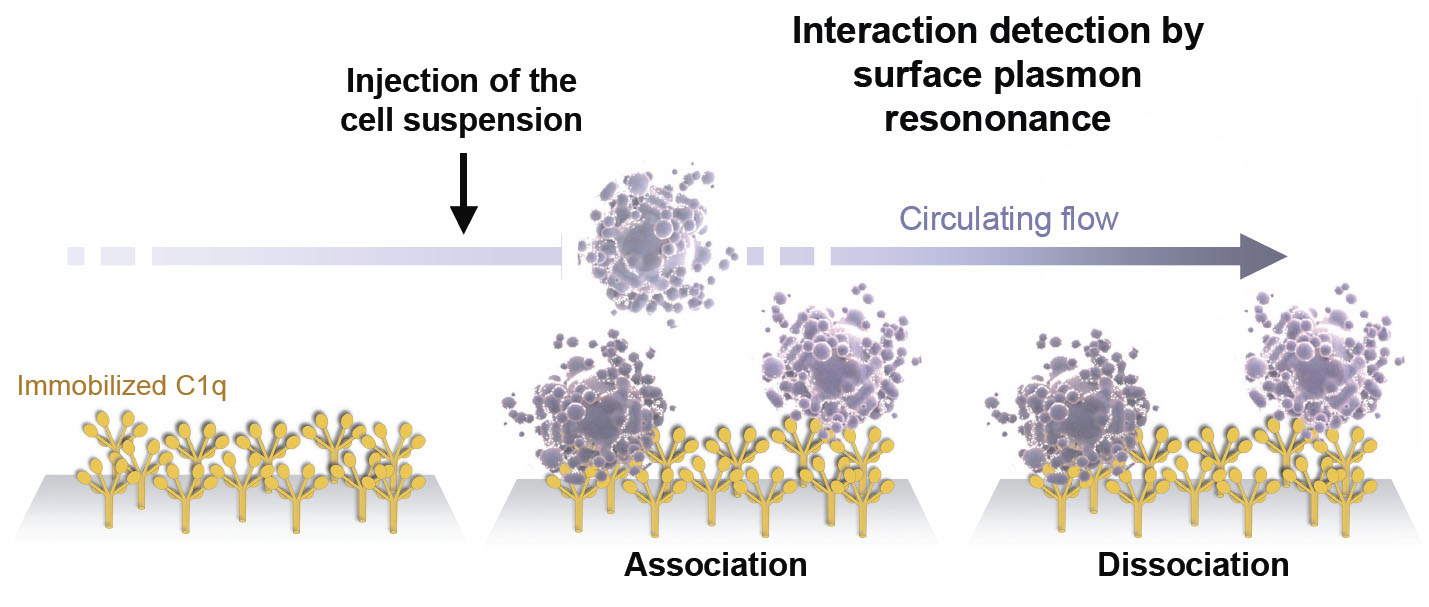
General principle of the BIAcore cellular assay
For this study, I first developed a cell-based BIAcore assay that allows to monitor interaction between C1q and cell surface, in real-time and without preliminary labeling (3). This assay allowed us to demonstrate that GR can bind to cell membrane rapidly after induction of apoptosis. By utilizing this assay, we showed that C1q GR specifically recognize different molecules on the surface of apoptotic cells.
The first goal of this work was to determine if one the known “eat-me” signals could be responsible for the specific apoptotic cell recognition by C1q.
• The first putative ligand that we tested was phosphatidylserine (PS), a canonical marker for apoptosis. Besides, PS in one of the best characterized “eat-me” signal, to date. By flow cytometry, we showed that early PS exposure takes place concomitantly with GR binding at the apoptotic cell surface. Furthermore, we demonstrated that C1q GR binds PS with high affinity. These results were extended with crystallographic studies, in collaboration with the laboratory for proteins crystallography and crystallogenesis from the IBS. We demonstrated that according to the structural model for C1q, this interaction is compatible with PS recognition at the surface of the target cell. Finally, using our cell-based BIAcore assay, we demonstrated the involvement of this interaction at the cellular level. Together, this state of the art multidisciplinary approach allowed us to identify the first ligand on apoptotic cells for C1q (3).
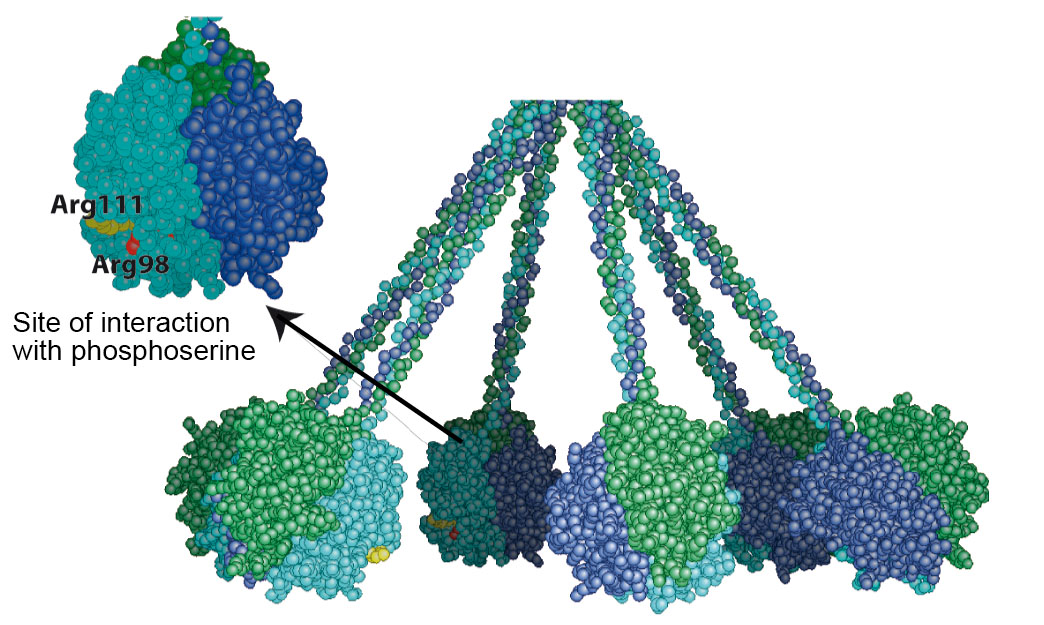
Cristallographic structure of the phosphoserine-GR complex
• The second part of this first project was motivated by previous results obtained in the lab showing that C1q has the ability to recognize immobilized glycoconjugates and that this lectine-like activity can be very efficiently inhibited by deoxy-D-ribose. Precisely, DNA has been characterized as an “eat-me” signal early exposed at the apoptotic cell surface (4). Using the same experimental strategy as for PS we demonstrated that DNA is also specifically recognized by C1q on the apoptotic cell surface, and that this recognition is specifically inhibited by deoxy-D-ribose (5).
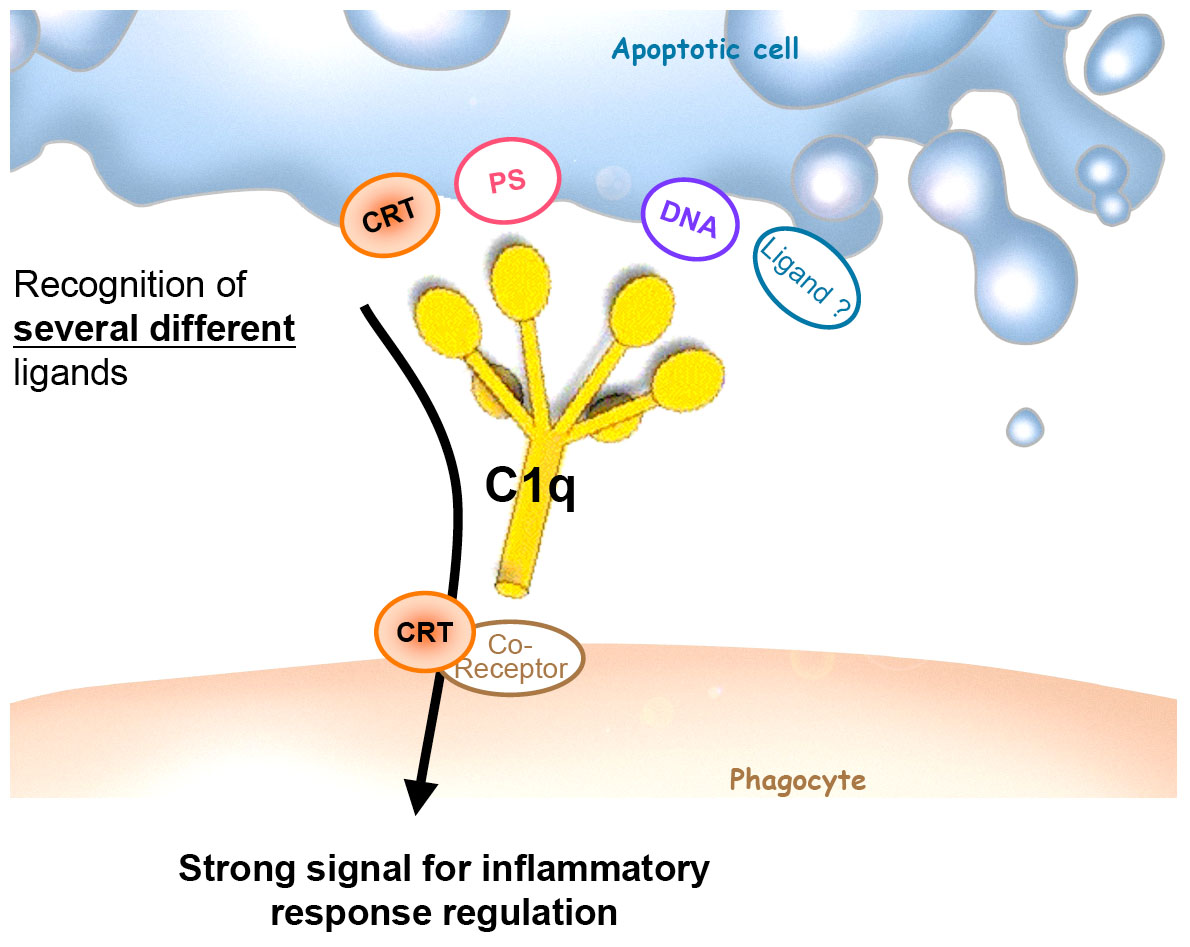
The second goal of my project was to determine if the known C1q receptors/partners could be potential ligands on the apoptotic cell surface. Calreticulin (CRT) has been recently implicated in apoptotic cell recognition as an “eat-me” signal at the apoptotic cell surface (6). It is admitted that CRT is able to recognize C1q CLF and that this interaction is involved at the phagocytic cell surface. However published studies regarding its interaction abilities with C1q GR are contradictory. Using the BIAcore, we demonstrated that GR also interact with high affinity with CRT. This result allows us to hypothesize that C1q recognizes CRT exposed on the apoptotic cell surface. This was reinforced by confocal microscopy experiments showing that CRT accumulates at the cell surface during apoptosis and that CRT patches are co-localized with GR. The physiological relevance of this interaction is currently in progress (7).
In conclusion, during my PhD, I identified at least two novel ligands for C1q on apoptotic cell surface, phosphatidylserine and DNA, and defined CRT as a very promising C1q ligand. Thus, due to its heterotrimeric and multivalent characteristics, one C1q molecule has the unique ability to collect several different recognition signal which, altogether provides a very strong recognition signal. Given its decisive role in the control of inflammation, these recognition properties might be of major importance in the regulation of the immune response.
Eventually, this study could allow not only to precise the mechanisms implicated in inflammatory diseases progression, but also to consider the development of new therapeutic for autoimmune pathologies such as lupus.
Helena Païdassi was supported by a contract for doctoral studies from CEA Grenoble and by an ATER from Joseph Fourier Univeristy ( Grenoble I).
Bibliographie:
- Païdassi H., Tacnet-Delorme P., Arlaud GJ., and Frachet P. 2009. How phagocyte track down and respond to apoptotic cells. Crit Rev Immunol. On press
- Navratil JS., Watkins SC., Wisnieski JJ., and Ahearn JM. 2001. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol 166:3231-3239
- Païdassi H., Tacnet-Delorme P., Garlatti V., Darnault C., Ghebrehiwet B., Gaboriaud C., Arlaud GJ., and Frachet P. 2008. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol 180:2329-2338
- Elward K., Griffiths M., Mizuno M., Harris CL., Neal JW., Morgan BP., and Gasque P. 2005. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. JBC. 280:36342-36354
- Païdassi H., Tacnet-Delorme P., Arlaud GJ., Thielens N., and Frachet P. 2008. The lectin-like activity of human C1q and its implication in DNA and apoptotic cell recognition. FEBS letters 582 ( 20): 3111-6
- Gardai SJ., McPhillips KA., Frasch SC., Janssen WJ., Starefeldt A., Murphy-Ullrich JE., Bratton DL., Oldenborg PA., Michalak M., and Henson PM. 2005. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321-334
- Païdassi H, Tacnet-Delorme P, Houen G, Arlaud GJ and Frachet P. Article en préparation sur le rôle de la calréticuline dans la reconnaissance des cellules apoptotiques par C1q