Home Page
GREMI Prize 2010, thesis summary:
TLR2 signaling, organization and variability of innate immune response
Julie TOUBIANA
This work was performed at :
Cochin Institute INSERM U1016 / CNRS UMR 8104
Host invasion by micro-organisms induces an innate immune response that leads to monocytes/macrophage cell activation, cytokine and chemokine expression and to the initiation of an adaptative response. Major differences are found within the organization of innate immune responses depending on the pathogen, the invaded cell-type and the host itself. This variability involves the regulation of pro-or anti‐inflammatory molecules, or the engagement of specific signaling pathways depending on the interactions between Toll like receptors (TLRs) (1). Initiation and organization of innate immune responses facing bacteria, parasites or fungi need the cooperation of TLR2 with TLR1 or TLR6 and multimolecular complexes in lipid rafts to determine a specific and a proper response (2). However, our understanding of molecular interactions within lipid rafts remains cryptic. Specifically, little is known regarding the composition of signaling complexes induced by distinct pathogens and the activation mechanisms involved in the initiation, amplification and regulation of a potent inflammatory response. The aim of this research project was to investigate the mechanisms involved in recognition and signaling downstream TLR2, and factors that account for variability of the inflammatory phenotype. For this purpose, we established a differential proteomic strategy to study the composition of TLR2 activation cluster and post-translationnal modifications of proteins after the recruitment of TLR2-TLR1 and TLR2-TLR6 to lipid rafts (Figure 1).
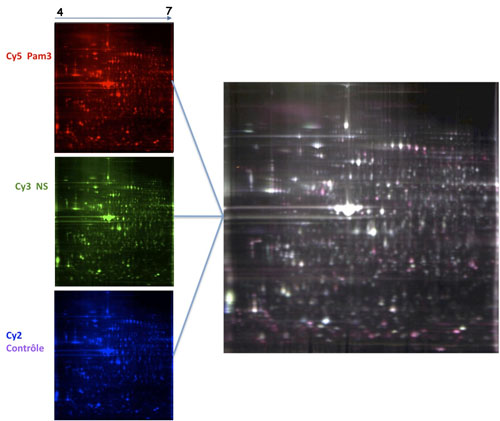
This approach enabled us to identify Lyn tyrosine‐kinase and IMPDHII protein (Inosine 5’ Monophosphate Dehydrogenase II) as part of TLR2 signaling (Figure 2 & 3). Lyn is essential for the activation of NF-κB following the engagement of TLR2 through the activation of PI-3kinase and subsequent transactivation of the NF-κB subunit p65 (Figure 2). IMPDHII was found to be a negative regulator of TLR2 signaling through the activation of SHP1 phosphatase and subsequent dephosphorylation of p85 subunit of PI3‐kinase (Figure 3). The exact mechanism involved remains to be explained (3).
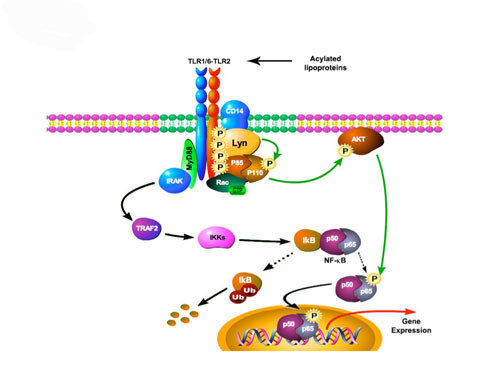
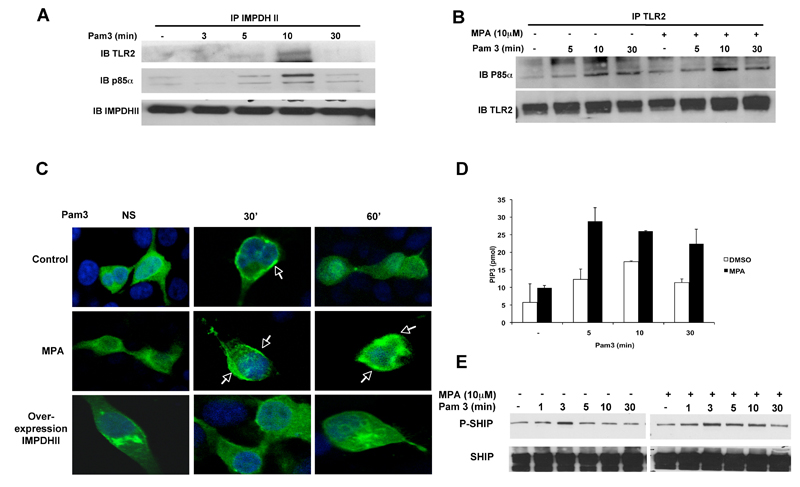
Figure 3. IMPDHII modulates PI3-K activation upstream of Akt. Activation of PI-3Kinase leads to the formation of PIP3. To investigate the role of IMPDHII on PI-3K activity, we transfected HEK293- T2 cells with a construct encoding a fusion protein between the PH domain of Akt and GFP, and studied translocation of Akt to the plasma membrane after stimulation with Pam3 with Confocal microscopy. In control conditions, Pam3 induced a transient translocation of the fluorescent probe to the plasma membrane 30 min after stimulation. Inhibition of IMPDHII activity with MPA resulted in a sustained recruitment of Akt to PIP3 whereas overexpression of IMPDHII prevented the recruitment of Akt to the cell membrane, suggesting that IMPDHII negatively regulates PI3-K
Finally, in a translational approach, we studied the effect of a functionally relevant haplotype of IRAK1 gene (which encodes for a key signaling protein downstream of most of the TLRs) on clinical variability in patients with septic shock. This work suggested that the IRAK1 variant haplotype (with gain of function) tagged by SNP IRAK1 1595C was associated with lung injury severity in a large cohort of more than 800 patients with septic shock (4). Our results provide new insights in organization, regulation and variability of innate immune response. These findings highlight the importance of balanced signals coordinating the inflammatory response to infection and may help to find novel targets in the control of TLR2-mediated sepsis.
- 1. Kawai, T., and Akira, S. (2010) Nat Immunol 11 , 373-384
- 2. Triantafilou, M., Gamper, F. G., Haston, R. M., Mouratis, M. A., Morath, S., Hartung, T., and Triantafilou, K. (2006) J Biol Chem 281 , 31002-31011
- 3. Toubiana, J., Rossi, A. L., Grimaldi, D., Belaidouni, N., Chafey, P., Clary, G., Courtine, E., Pene, F., Mira, J. P., Claessens, Y. E., and Chiche, J. D. (2011) J Biol Chem
- 4. Toubiana, J., Courtine, E., Pene, F., Viallon, V., Asfar, P., Daubin, C., Rousseau, C., Chenot, C., Ouaaz, F., Grimaldi, D., Cariou, A., Chiche, J. D., and Mira, J. P. (2010) Crit Care Med 38 , 2287-2294